DESIGNED WITH RADIAL ACCESS
TO THE PERIPHERY IN MIND
The Sublime™ Radial Access Guide Sheath is the industry’s first and only radial length 5 Fr sheath, available in 5 Fr and 6 Fr, 120 cm and 150 cm.
Reliable Access and Delivery
The Sublime guide sheath is constructed with a proprietary braided shaft technology that combines the best performance features of traditional coil and braid structures in a thin-walled profile. The result: best-in-class kink resistance, torque response, and radial strength.
GUIDE SHEATH CROSS-SECTION

Full length Serene™ hydrophilic coating designed to minimize vessel damage and spasm while optimizing trackability through distal tortuosity

Polymer outer jacket provides uniform device structure

PTFE-lined inner lumen for smooth device passage and easy device tracking to distal target lesions

Proprietary braided shaft technology designed to offer maximum kink resistance, torque response, and radial strength
The First True 5 Fr Radial Length Guide Sheath
Size matters for radial sheaths. In a randomized, multicenter study, use of the Terumo Destination Slender™ 6 Fr sheath versus a standard 5 Fr sheath
(OD .090 in) was associated with a 2-fold higher rate of radial artery occlusion (3.47% vs 1.73%, respectively).*
RADIAL LENGTH SHEATH OUTER DIAMETERS (IN)
Note: OD Measurements sourced from respective product brochures.
* Aminian A, Saito S, Takahashi A, et al. Comparison of a new slender 6 Fr sheath with a standard 5 Fr sheath for transradial coronary angiography and intervention: RAP and BEAT (Radial Artery Patency and Bleeding, Efficacy, Adverse evenT), a randomised multicentre trial. EuroIntervention. 2017;13(5):e549-e556.
“The availability of the Sublime 5 Fr guide sheath allows us to perform radial interventions on patients with smaller arteries—older female patients, for example—who otherwise would require femoral access.”
Ankur Lodha, MD
Cardiovascular Institute of the South
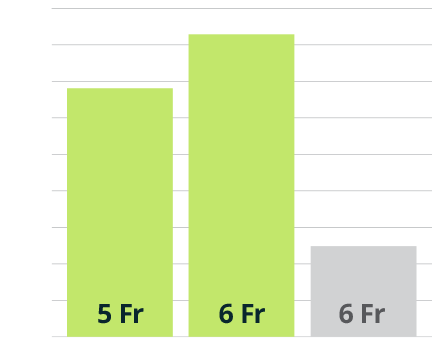
Sublime Guide Sheath Versus the Competition
Competitive bench testing demonstrates the Sublime guide sheath is an ideal solution for delivering devices to the lower extremities, while providing better kink resistance, torque response, and radial strength than the Terumo Destination Slender™ 6 Fr sheath.

5 Fr and 6 Fr Sublime™ Radial Access Guide Sheath

Terumo R2P™ Destination Slender™ 6 Fr Guiding Sheath
Better Kink Resistance1
Lower number is better
Better Torque Response1
Higher number is better
Better Radial Strength1
Higher number is better
Comparison Testing
- Average measurements from bench testing by Surmodics, Inc. R2P™ Destination Slender™ Guiding Sheath, 6 Fr (Terumo Medical Corp.) N=5; Sublime™ Radial Access Guide Sheath, 5 Fr (Surmodics, Inc.) N=5; Sublime™ Radial Access Guide Sheath, 6 Fr (Surmodics, Inc.) N=5. Data on file. Bench test results may not necessarily be indicative of clinical performance.
Testing Method
Kink Resistance: Each test device was made into a loop and placed into a kink resistance test fixture mandrel. Visual inspection of kink was administered. If no kink occurred, the devices were made into a smaller loop and placed in the mandrel. This was continued until a kink was visually observed on the test device and the mandrel radius at which the kink occurred was recorded.
Torque: Each test device was placed into calibrated torque test instrument. Devices were clamped into the torque test instrument on the distal end and rotated via the proximal hub. Devices were rotated clockwise one full rotation and maximum torque force on torque sensor was recorded.
Radial Strength: Each test device was compressed between two flat plates. Force was measured at a fixed percent compression of the shaft diameter.
Purpose-designed for transradial-to-peripheral interventions, the Sublime guide sheath is the industry’s first and only radial length 5 Fr sheath, available in 5 Fr and 6 Fr, 120 cm and 150 cm.
Distance based on 5’9” patient height, measurements are approximate
Product Features

Available in 5 Fr and 6 Fr, 120 cm and 150 cm lengths

Hemostasis valve with side arm for flushing

Radiopaque markers on guide sheath and radiopaque dilator increase visibility under fluoroscopy

Packaged with an .018” or .035” dilator

Smooth tip transitions designed for atraumatic vessel entry
Product Matrix
| 8
Sizes |
120 cm & 150 cm
Working Length |
5 Fr
Diameter Sheath ID 0.076" (1.9 mm) Sheath OD 0.089" (2.3 mm) |
6 Fr
Diameter Sheath ID 0.088" (2.2 mm) Sheath OD 0.101" (2.6 mm) |
.018" & .035"
Guidewire Compatible Dilator |
|---|
| Model Number | Sheath Length | French Size | Sheath ID | Sheath OD | Guidewire |
|---|---|---|---|---|---|
| SRA-GS18-5F120 | 120 cm | 5 Fr | 0.076” / 1.9 mm | 0.089” / 2.3 mm | 0.018” |
| SRA-GS18-5F150 | 150 cm | 5 Fr | 0.076” / 1.9 mm | 0.089” / 2.3 mm | 0.018” |
| SRA-GS18-6F120 | 120 cm | 6 Fr | 0.088” / 2.2mm | 0.101” / 2.6 mm | 0.018” |
| SRA-GS18-6F150 | 150 cm | 6 Fr | 0.088” / 2.2mm | 0.101” / 2.6 mm | 0.018” |
| SRA-GS35-5F120 | 120 cm | 5 Fr | 0.076” / 1.9 mm | 0.089” / 2.3 mm | 0.035” |
| SRA-GS35-5F150 | 150 cm | 5 Fr | 0.076” / 1.9 mm | 0.089” / 2.3 mm | 0.035” |
| SRA-GS35-6F120 | 120 cm | 6 Fr | 0.088” / 2.2mm | 0.101” / 2.6 mm | 0.035” |
| SRA-GS35-6F150 | 150 cm | 6 Fr | 0.088” / 2.2mm | 0.101” / 2.6 mm | 0.035” |
| 8
Sizes |
|---|
| 5 Fr
Diameter Sheath ID 0.076" (1.9 mm) Sheath OD 0.089" (2.3 mm) |
6 Fr
Diameter Sheath ID 0.088" (2.2 mm) Sheath OD 0.101" (2.6 mm) |
|---|
| 120 cm & 150 cm
Working Length |
|---|
| .018" &.035"
Guidewire Compatible Dilator |
|---|
| Model Number | Sheath Length | French Size | Sheath ID | Sheath OD | Guidewire |
|---|---|---|---|---|---|
| SRA-GS18-5F120 | 120 cm | 5 Fr | 0.076” / 1.9 mm |
0.089” / 2.3 mm |
0.018” |
| SRA-GS18-5F150 | 150 cm | 5 Fr | 0.076” / 1.9 mm |
0.089” / 2.3 mm |
0.018” |
| SRA-GS18-6F120 | 120 cm | 6 Fr | 0.088” / 2.2mm |
0.101” / 2.6 mm |
0.018” |
| SRA-GS18-6F150 | 150 cm | 6 Fr | 0.088” / 2.2mm |
0.101” / 2.6 mm |
0.018” |
| SRA-GS35-5F120 | 120 cm | 5 Fr | 0.076” / 1.9 mm |
0.089” / 2.3 mm |
0.035” |
| SRA-GS35-5F150 | 150 cm | 5 Fr | 0.076” / 1.9 mm |
0.089” / 2.3 mm |
0.035” |
| SRA-GS35-6F120 | 120 cm | 6 Fr | 0.088” / 2.2mm |
0.101” / 2.6 mm |
0.035” |
| SRA-GS35-6F150 | 150 cm | 6 Fr | 0.088” / 2.2mm |
0.101” / 2.6 mm |
0.035” |
Indications for Use: The Guide Sheath is intended to introduce therapeutic or diagnostic devices into the vasculature, excluding the coronary and neuro vasculature. Note: The Sublime Radial Access Guide Sheath indications do not specify access puncture site. The access site is at the discretion of the physician.
Third-party trademarks are the property of their respective owners.